Topical Finasteride, the new hairloss medication?
Finasteride is a widely used treatment for androgenetic alopecia (AGA)—the most common form of hair loss in men and women. Despite its effectiveness for male pattern baldness, it has been associated with various side effects including erectile dysfunction, decreased libido, abnormal ejaculation, insomnia, and depression.1 A topical version of the drug is currently being studied, as scientists hope to lower the incidence of side effects through a new delivery method.
Topical finasteride is not yet available commercially, but you may get it from compounding pharmacies. However, you should know that only topical minoxidil and oral finasteride were approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of male pattern baldness.2 The efficacy and safety of finasteride in other forms are yet to be established.
While some of you eagerly await the approval and availability of topical finasteride, let’s discuss what androgenetic alopecia is, how finasteride works, and what are the side effects reported by people who actually used it.
What is Androgenetic Alopecia?

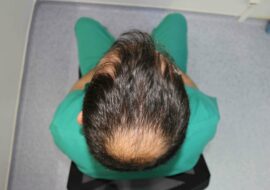
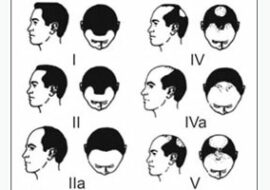
There are different types of hair loss that occur in both sexes. Androgenetic alopecia, also known as male pattern baldness or female pattern baldness, is the most common type of alopecia in men and women. It affects at least 30% of men by the age of 30 years and about 50% by the age of 50. While its prevalence is lower in women than in men, it still occurs to a great extent.
In men, hair-thinning starts at the temples and head’s crown, forming an M-shaped pattern. Women, on the other hand, experience hair loss, which starts in the central and frontal scalp with retention of the frontal hairline. As time passes, hair thinning may lead to complete baldness.
It is understandable how this condition affects an individual’s self-esteem. Healthy hair signifies youth and vitality, and people suffering from visible hair loss are often seen as old looking and less physically attractive. The prevalence of androgenetic alopecia and its impact on the patient’s social and psychological well-being is the reason why there’s a huge market for hair loss treatments.
The downside is that current pharmacological treatments are not entirely safe and the benefits are not maintained upon ceasing therapy. Studies have confirmed that finasteride can cause sexual and psychological side effects. There are also some evidence suggesting that such adverse effects can continue for a long time even if you stop taking the medication.
Finasteride: hair loss treatment for men
Finasteride was approved by the FDA in 1997 for male pattern baldness or hereditary hair loss.3 It is currently being sold under the brand names Propecia, Proscar, and Aindeem to name a few. Initially, the drug was only marketed to treat benign prostatic hyperplasia (BPH) or enlarged prostate in men aged 60 and above. By some sort of luck, researchers found that treated patients did not present with receding hairlines, which prompted large-scale clinical trials to test the effectiveness of finasteride for AGA. Right now, it is also being used off-label (unapproved indication) to treat hirsutism or excessive hair growth in women, as well as a hormone replacement therapy for transgender women.
However, this medication is only approved for MEN with AGA. It is not recommended to treat female pattern hair loss, especially in women of childbearing potential, as it can cause abnormalities to the external genitalia of the male fetus. In fact, the drug label for oral finasteride (Propecia®) states, “women who are pregnant or may become pregnant should not handle crushed or broken PROPECIA tablets.”4 Additionally, studies have found it ineffective in treating AGA in women.5
With that being said, it’s not illegal to prescribe finasteride to women with hair loss. As I’ve mentioned above, certain medications may be prescribed to treat conditions that has not been included in its indications; it’s called off-label usage, and it depends on the discretion of the prescribing physician.
How does Finasteride work?
It has been found that AGA does not occur in individuals with a genetic deficiency of the type 2 5-alpha-reductase, an enzyme essential in converting the male sex hormone testosterone to the more potent dihydrotestosterone (DHT). Increased levels of DHT are said to be responsible in causing hair loss, but it’s not the presence of DHT alone that should be addressed. Men who are predisposed to AGA have an increased sensitivity to DHT. The genetically predisposed hair follicles in these men are prone to hair miniaturization, which leads to progressive decline in scalp hair density.
HAIR FOLLICLE MINIATURIZATION
Normal terminal hairs that are large, thick, and pigmented are converted into thin, barely visible, depigmented hairs called vellus hairs.
Finasteride is a synthetic compound that blocks the activity of 5-alpha-reductase enzyme, causing reduction of scalp DHT by 64% and serum DHT by 68%.6 This makes the drug effective in reducing the size of an enlarged prostate. DHT may be the culprit in male pattern hair loss; however, it is also an important hormone for the formation of sexual characteristics during puberty. This means finasteride should not be used by men below the age of 18.
Safety and Efficacy of Finasteride according to Studies
Is finasteride effective in treating hair loss? The mere fact that finasteride was granted an FDA and EMA approval means experts have found the drug effective for male pattern baldness. The therapeutic dose for treating AGA is 1 mg tablet to be taken by mouth once daily and 5 mg for enlarged prostate. When Merck launched Propecia for male pattern baldness in the late 90s, it was a blockbuster drug. Back then, the label only warned that about 1% of men might experience erectile dysfunction and low libido.
It did not warn about other possible forbidding symptoms such as insomnia, depression, or suicidal ideation. What’s even worse was their claim that-- “these (sexual) side effects went away in men who stopped taking Propecia.” It turns out that the bad effects of the drug stay longer in some individuals.
A 2015 review, published in JAMA Dermatology, found that out of 34 clinical trial reports for finasteride, none had adequately assessed the safety of the drug.7 It is troubling to know that millions of patients had been using finasteride for years before the company decided to update the drug’s label in 2012. It has since added depression to the label and acknowledge patients’ reports that the drug’s sexual side effects may continue even after discontinuation of treatment.
So once you decide to stop taking the drug, hair loss comes back and side effects may also remain. No matter how many times you toss the coin, it looks like you are fighting a losing battle.
This data raises the question of why would anyone choose to take a medication that can possibly
Here’s another thing to consider:
Normal terminal hairs that are large, thick, and pigmented are converted into thin, barely visible, depigmented hairs called vellus hairs.8
affect sexual functioning and cause depression in exchange of a not-so-stellar outcome? After all, androgenetic alopecia and benign prostatic hyperplasia are non-life-threatening conditions.
The Post-Finasteride Syndrome
More than 1,300 lawsuits were filed against Merck, as patients claimed that the symptoms of erectile dysfunction, loss of libido, reduction in penis size, cognitive impairment, and depression severely affected their families, relationships, and careers—even drove some people to take their own lives. Cases of sexual side effects, depression, and suicide had been alarming that patients coined the term post-finasteride syndrome, referring to the group of symptoms that patients experience even after stopping treatment.9
The non-profit organization, the Post-Finasteride Syndrome Foundation (www.pfsfoundation.org), is dedicated in supporting finasteride patients and improving public awareness of the condition. Physicians are now advised to refrain from prescribing oral finasteride to patients with a history of sexual dysfunction, fertility problems, and depression.9
Common and Uncommon Side Effects
Now let’s take a look at side effects and risks associated with finasteride use. The adverse effects from the two studies are presented in order of decreasing incidence.
| PLESS Study PROSCAR Long-Term Efficacy and Safety Study (4-year Placebo controlled study) | MTOPS Study Medical Therapy of Prostatic Symptoms Study | Post-marketing Experience |
| Erectile dysfunction Decreased libido Decreased volume of ejaculate Ejaculation problems Breast enlargement Breast tenderness Rash | Erectile dysfunction Dizziness Postural hypotension Asthenia Decreased libido Abnormal ejaculation Headache Somnolence Sexual function abnormality Peripheral edema Hypotension Dyspnea Breast enlargement Rhinitis | Hypersensitivity reactions: rash, itching, urticarial, swelling of the lips and face |
| Breast disorder: breast tenderness and enlargement (gynecomastia) | ||
| Malignancy: male breast cancer | ||
| Reproductive system: (sexual dysfunction that continued after treatment discontinuation) erectile dysfunction; libido, ejaculation, arousal, and orgasm disorders; male infertility, and testicular pain | ||
| Nervous: insomnia, depression, suicidal thinking |
As with all medications, side effects differ from person to person. Some patients may experience mild and self-limiting symptoms. Other may experience severe symptoms that persist even after treatment discontinuation.
A group of researchers also found some uncommon side effects associated with finasteride use. The subjects were treated with 1 mg finasteride for at least four months. A few patients experienced the following side effects:10
- Melasma (1 patients)
- Dry/thinning of skin (4 patients)
- Tinnitus (3 patients)
- Postural hypotension (2 patients)
- Oral lesions (9 patients)
Is Topical Finasteride effective?
Polichem, a Swiss pharmaceutical company, has been developing a topical version of finasteride. The drug under trial (P-3074) contains 0.25 percent finasteride versus the 1 mg found in tablet form. It is currently under Pivotal Phase III in EU,11 but the company has yet to announce the results from these studies.
In earlier studies, researchers claimed that topical finasteride induced systemic decrease in DHT levels at varying dose regimen with no side effects.12 However, the study treatment only lasted for a week, as opposed to the long term treatments commonly done in clinical practice. There was also a conflict of interest since the company, which funded the study is also developing the drug in question.
There is no general consensus over the safety and efficacy of topical finasteride for male pattern baldness. As topical drugs can still be absorbed systemically, it is better to err on the side of caution.
Finasteride alternative
The worrisome side effects associated with finasteride is enough for anyone to reconsider taking the drug. Does the benefits outweigh the risks? There are other approved drug therapies for AGA. However, even though most of them can stop the progression of hair loss, none can promise a permanent cure.
| Other Drug Therapies for Androgenetic Alopecia13 | |||
|---|---|---|---|
| Drug Name | Approval | Class | Route |
| Minoxidil (Rogaine) | Approved (male and female) | Vasodilator | Topical |
| Pinacidil (Pindac) | Clinical Trial | Vasodilator | Topical |
| Tretinoin (Retin-A) | Clinical Trial | Retinoid | Topical |
| Dutasteride (Avodart) | Approved (male) | 5-α-R inhibitor | Oral |
| Bicalutamide (Casodex) | Clinical Trial | Nonsteroidal anti-androgen | Oral |
| Fluridil (Eucapil) | Clinical Trial | Nonsteroidal anti-androgen | Topical |
| Eclipta alba extract | Animal Trial (mice) | Botanical extract | Topical |
| Antisense oligonucleotides | Investigational | -- | Topical |
Natural supplements, diet modifications, and combination therapies are also worth considering if you want to avoid the ugly side effects of finasteride. In some cases, male pattern baldness may also be due to an underlying medical condition. In one study, AGA have been linked to obesity or being overweight.
Inadequate food intake and personal food choices may lead to dietary deficiencies. Some micronutrients that are relevant to hair growth include:
- B vitamins (biotin, niacin, folic acid, riboflavin, pantothenic acid, pyridoxine, and cyanocobalamin)
- Antioxidants (vitamin A and E)
- Trace metals (iron, zinc, and selenium)
Hair loss does not often result from a single cause, but from a combination of several factors that must be addressed altogether for treatment success. To manage male pattern baldness, it is important for patients and clinicians to be aware of the variety of current therapeutic options available for them.
References:
1. Mella, J. M., Perret, M. C., Manzotti, M., Catalano, H. N., & Guyatt, G. (2010). Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Archives of Dermatology, 146(10). https://doi.org/10.1001/archdermatol.2010.256
2. Won Lee, Sung & Juhasz, Margit & Mobasher, Pezhman & Ekelem, Chloe & Atanaskova Mesinkovska, Natasha. (2018). A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. Journal of drugs in dermatology : JDD. 17. 457-463.
3. Trüeb, R. M. (2015). The difficult hair loss patient guide to successful management of alopecia and related conditions. Cham: Springer International Publishing
4. Merck & Co.,Inc. (1992). PROPECIA® (finasteride) tablets for oral use [Ebook]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf
5. Price, V. H., Roberts, J. L., Hordinsky, M., Olsen, E. A., Savin, R., Bergfeld, W., Waldstreicher, J. (2000). Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. Journal of the American Academy of Dermatology, 43(5, Part 1), 768–776. https://doi.org/10.1067/mjd.2000.107953
6. Cranwell, W., & Sinclair, R. (2000). Male androgenetic alopecia. In K. R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, K. Dungan, A. Grossman, D. P. Wilson (Eds.), Endotext. South Dartmouth (MA): MDText.com, Inc. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK278957/
7. Belknap, S. M., Aslam, I., Kiguradze, T., Temps, W. H., Yarnold, P. R., Cashy, J., West, D. P. (2015). Adverse event reporting in clinical trials of finasteride for androgenic alopecia: a meta-analysis. JAMA Dermatology, 151(6), 600–606. https://doi.org/10.1001/jamadermatol.2015.36
8. English, R. S. (2018). A hypothetical pathogenesis model for androgenic alopecia: clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Medical Hypotheses, 111, 73–81. https://doi.org/10.1016/j.mehy.2017.12.027
9. Trüeb, R. M., & Lee, W.-S. (2014). Male alopecia: guide to successful management. Cham ; New York: Springer.
10. Georgescu, Simona Roxana & Paunica, S & Tampa, Mircea & Sarbu, M.I. & Limbau, A.M. & Paunica, I & Baleanu, B.C. & Motofei, Ion. (2016). 200 Uncommon finasteride side effects in male androgenic alopecia. Journal of Investigative Dermatology. 136. S195.10.1016/j.jid.2016.06.219.
11. Polichem - Research and Development. (2019). Retrieved from http://www.polichem.com/polichem/rd.html
12. Caserini, M., Radicioni, M., Leuratti, C., Annoni, O., & Palmieri, R. (2014). A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. Int. Journal of Clinical Pharmacology and Therapeutics, 52(10), 842–849. https://doi.org/10.5414/CP202119
13. Santos, Z., Avci, P., & Hamblin, M. R. (2015). Drug discovery for alopecia: gone today, hair tomorrow. Expert opinion on drug discovery, 10(3), 269-92.